-
 South Korea president clings to power after martial law U-turn
South Korea president clings to power after martial law U-turn
-
Presidential vote seen as referendum on Romania's European future

-
 Hamilton bids farewell to Mercedes as Ferrari vie for title
Hamilton bids farewell to Mercedes as Ferrari vie for title
-
New Zealand unchanged in bid to hit back against England

-
 Macron seeks remedy to France's political crisis
Macron seeks remedy to France's political crisis
-
New Natalia Lafourcade album celebrates music's onstage evolutions

-
 Taiwan's Lai kicks off visit to US territory Guam
Taiwan's Lai kicks off visit to US territory Guam
-
Ivory Coast staple cassava meal gains UNESCO heritage status

-
 OpenAI to partner with military defense tech company
OpenAI to partner with military defense tech company
-
Liverpool held but Slot salutes 'special' Salah

-
 Man City needed to break losing 'routine', says Guardiola
Man City needed to break losing 'routine', says Guardiola
-
Leipzig down Frankfurt to reach German Cup quarters, Cologne strike late

-
 Mbappe admits penalty miss 'big mistake' as Bilbao beat Real Madrid
Mbappe admits penalty miss 'big mistake' as Bilbao beat Real Madrid
-
'Sad, disappointed' Mbappe pays penalty as Bilbao beat Real Madrid

-
 US stocks surge to records, shrugging off upheaval in South Korea, France
US stocks surge to records, shrugging off upheaval in South Korea, France
-
Liverpool held in Newcastle thriller, Arsenal inflict Amorim's first defeat

-
 Shiffrin confirms she'll miss Beaver Creek World Cup races
Shiffrin confirms she'll miss Beaver Creek World Cup races
-
Corner kings Arsenal beat Man Utd to close gap on Liverpool

-
 Mbappe pays penalty as Bilbao beat Real Madrid
Mbappe pays penalty as Bilbao beat Real Madrid
-
NFL Jaguars place Lawrence on injured reserve with concussion

-
 North Korea, Russia defence treaty comes into force
North Korea, Russia defence treaty comes into force
-
Openda hits brace as Leipzig beat Frankfurt in German Cup last 16

-
 Schar punishes Kelleher blunder as Newcastle hold Liverpool in thriller
Schar punishes Kelleher blunder as Newcastle hold Liverpool in thriller
-
De Bruyne masterclass helps Man City end seven-game winless streak

-
 Syrian rebels surround Hama 'from three sides', monitor says
Syrian rebels surround Hama 'from three sides', monitor says
-
Lawyers seek leniency for France rape trial defendants, blaming 'wolf' husband

-
 OpenAI chief 'believes' Musk will not abuse government power
OpenAI chief 'believes' Musk will not abuse government power
-
Thousands rally in Georgia after police raid opposition offices

-
 S. Korea opposition push to impeach president
S. Korea opposition push to impeach president
-
Powell 'not concerned' US Fed would lose independence under Trump

-
 French government falls in historic no-confidence vote
French government falls in historic no-confidence vote
-
Syrian White Helmets chief 'dreams' of never pulling a body out of rubble again

-
 NBA Suns lose Durant for at least a week with ankle injury
NBA Suns lose Durant for at least a week with ankle injury
-
Warhammer maker Games Workshop enters London's top stocks index

-
 Iran Nobel winner released for three weeks, 'unconditional' freedom urged
Iran Nobel winner released for three weeks, 'unconditional' freedom urged
-
Red Cross marks record numbers of humanitarians killed in 2024

-
 Johnson's Grand Slam 'no threat', says World Athletics boss Coe
Johnson's Grand Slam 'no threat', says World Athletics boss Coe
-
Qatar's emir and UK's Starmer talk trade as state visit ends

-
 Cuba suffers third nationwide blackout in two months
Cuba suffers third nationwide blackout in two months
-
Russia, Ukraine to send top diplomats to OSCE summit in Malta

-
 Spanish royals to attend memorial service for flood victims
Spanish royals to attend memorial service for flood victims
-
LPGA, USGA new policy requires female at birth or pre-puberty change

-
 Stick to current climate change laws, US tells top UN court
Stick to current climate change laws, US tells top UN court
-
British Museum chief says Marbles deal with Greece 'some distance' away

-
 Pope Francis receives electric popemobile from Mercedes
Pope Francis receives electric popemobile from Mercedes
-
Gaza civil defence: thousands flee Israeli strikes, evacuation calls

-
 Trump names billionaire private astronaut as next NASA chief
Trump names billionaire private astronaut as next NASA chief
-
Pidcock to leave INEOS Grenadiers at end of season

-
 Seoul stocks weaken, Paris advances despite political turmoil
Seoul stocks weaken, Paris advances despite political turmoil
-
South America summit hopes to seal 'historic' trade deal with EU


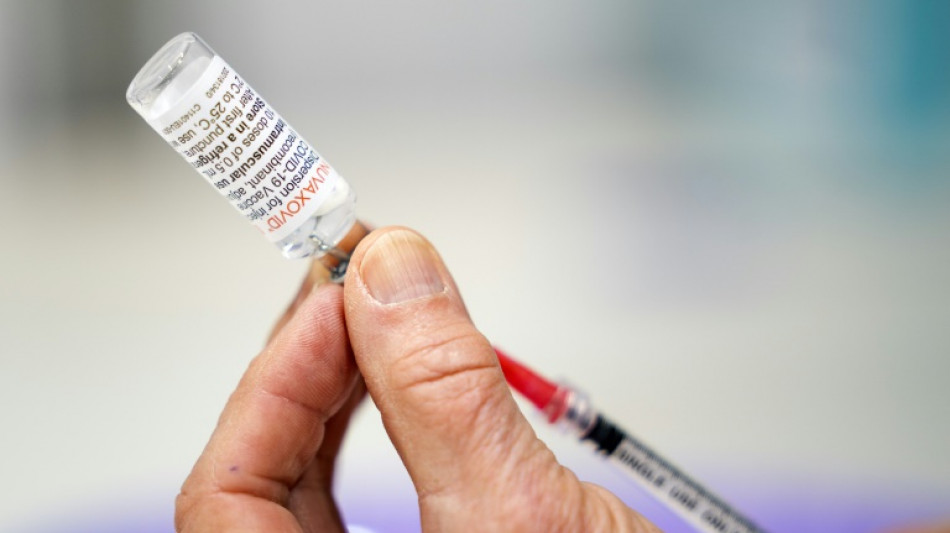
US experts recommend Novavax Covid-19 vaccine
A panel of experts convened by the US drug regulator on Tuesday recommended the Novavax Covid-19 shot, a late runner in the fight against the virus that could nonetheless play a role in overcoming vaccine hesitancy.
Three vaccines are currently approved in the United States: Pfizer and Moderna, which are based on messenger RNA, and Johnson and Johnson, which recently received a recommendation against broad use becase of links to a serious form of clotting.
Experts voted 21 in favor of the Novavax vaccine, with none against, and one abstention, despite some concerns it may be linked to rare cases of heart inflammation.
The Food and Drug Administration, which called the meeting, is expected to issue an emergency use authorization soon. Then another agency, the Centers for Disease Control and Prevention, will weigh in with guidance on how it should best be used.
Maryland-based Novavax was an early frontrunner in the global vaccine race, but fell behind after being hit by manufacturing and regulatory delays.
The US was one of the few major markets where it hasn't yet received authorization, while the EU, UK, Canada, Australia are among many that have already given it the green light.
Officials hope that the shot, which is based on lab-grown viral proteins, could provide an alternative for people still hesitant of the mRNA technology. It also doesn't have the same cold storage requirements as Pfizer and Moderna's shots.
"There really is a population of patients who are willing to take this and not going to take existing vaccines. I think it's pretty compelling," said Eric Rubin, an infectious disease specialist who participated in the meeting, explaining his vote in favor.
Of the various vaccine technologies, mRNA has been subject to the most misinformation efforts.
- Possible myocarditis link -
Novavax's vaccine was found to be more than 90 percent effective against symptomatic cases of the disease. But its trial was conducted long before the currently circulating sub variants of Omicron were dominant, and the company may yet have to add a booster or update its shot.
What's more, six cases of myocarditis, an inflammation of the heart muscle, were detected in the group that received the vaccine, against one case in the placebo group, in a trial of around 40,000 people.
Novavax says there is insufficient evidence to establish a causal relationship between the cases of myocarditis and the vaccine.
Such a link has been established with mRNA vaccines, but it only became apparent when they were used on millions of people in the real world, rather than tens of thousands in a trial.
The FDA voiced concern over the myocarditis link on Friday, and a warning is likely to be included on the eventual label. Earlier, trading in Novavax shares on Nasdaq was halted pending the meeting.
Known as a protein subunit vaccine, Novavax is administered in two doses.
It is based on a lab-created version of the spikes that dot the surface of the coronavirus to evoke an immune response.
The company uses a modified spike gene inserted into another kind of virus, called a baculovirus, which is used to infect moth cells, which then produce the spikes on their surface. These spikes are harvested and assembled into nanoparticles, which are injected into patients.
A compound of soapbark tree is added to the vaccine to heighten the response.
C.Meier--BTB




